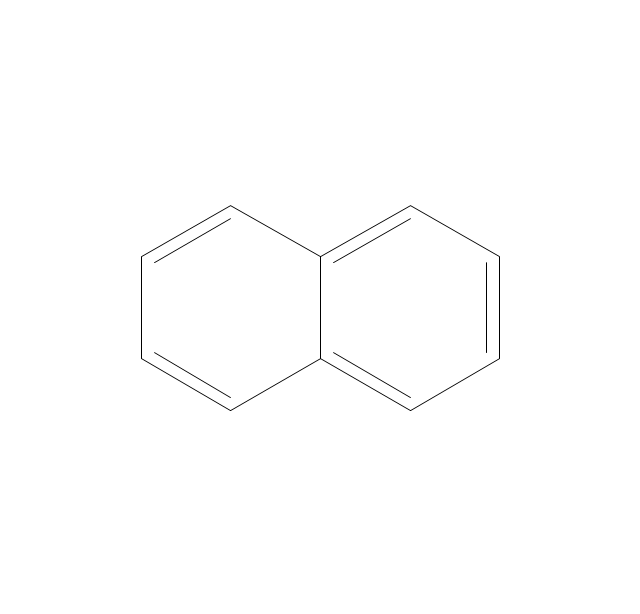
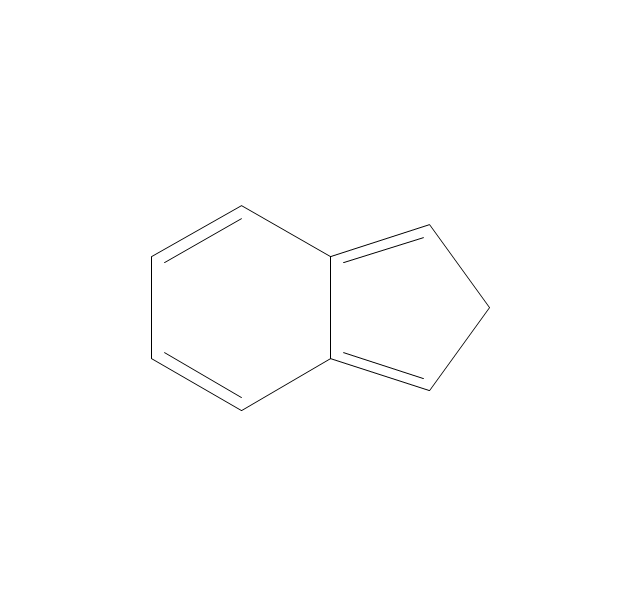
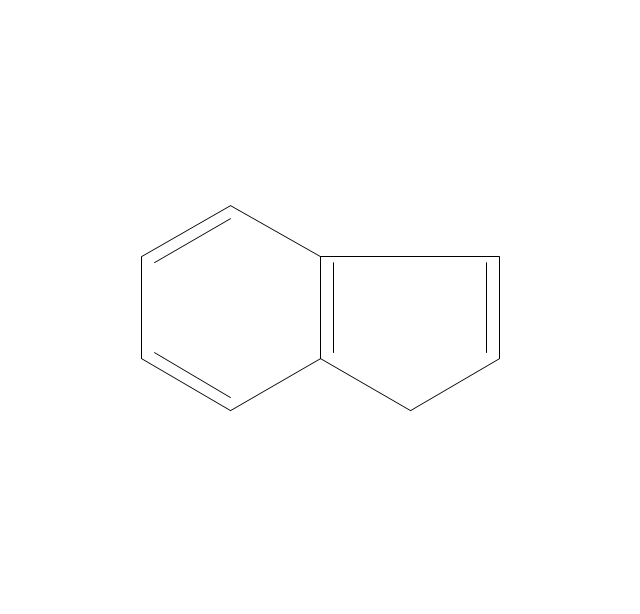
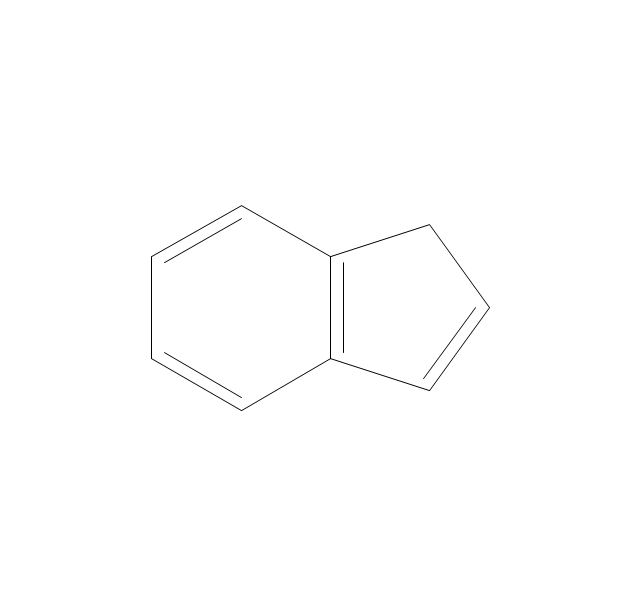
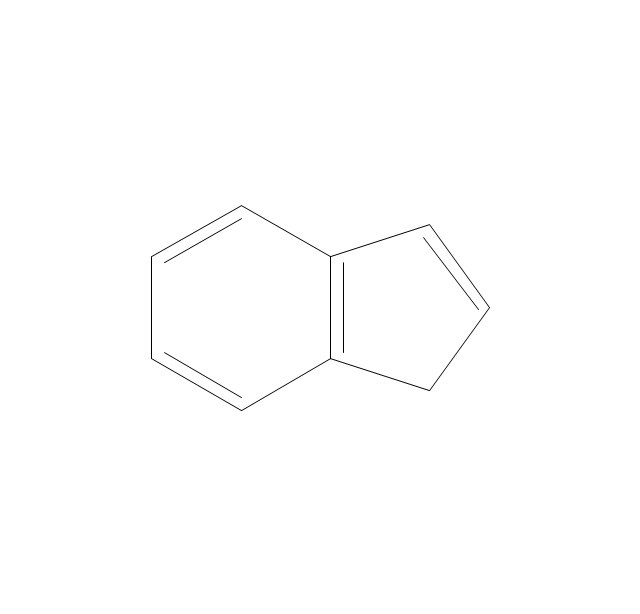
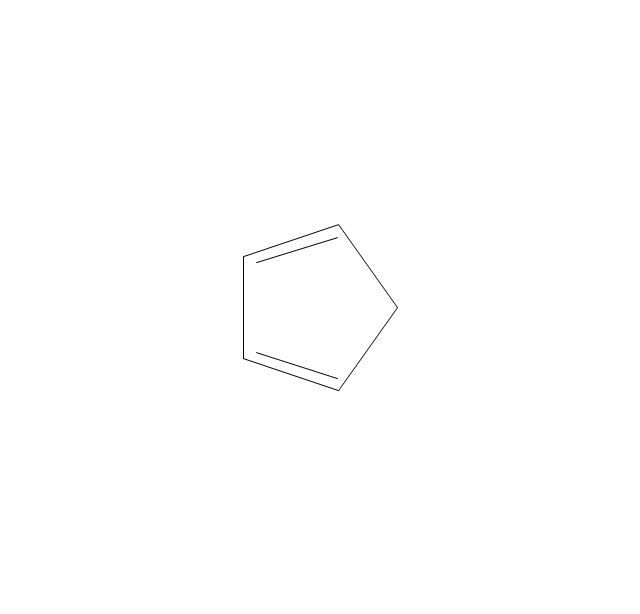
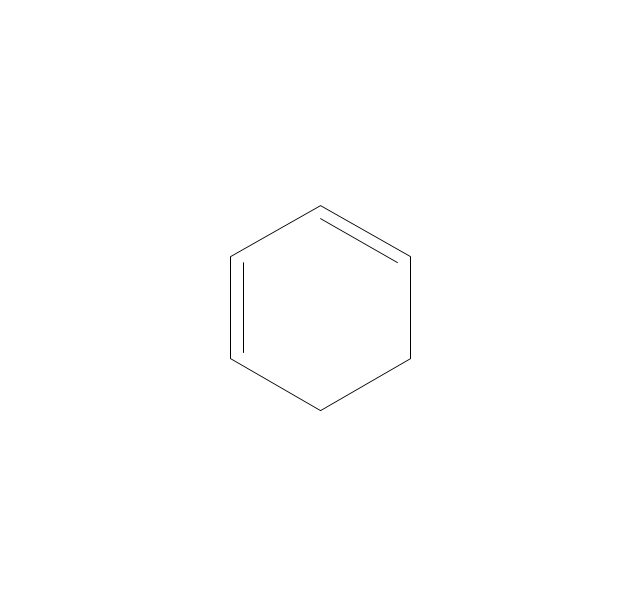
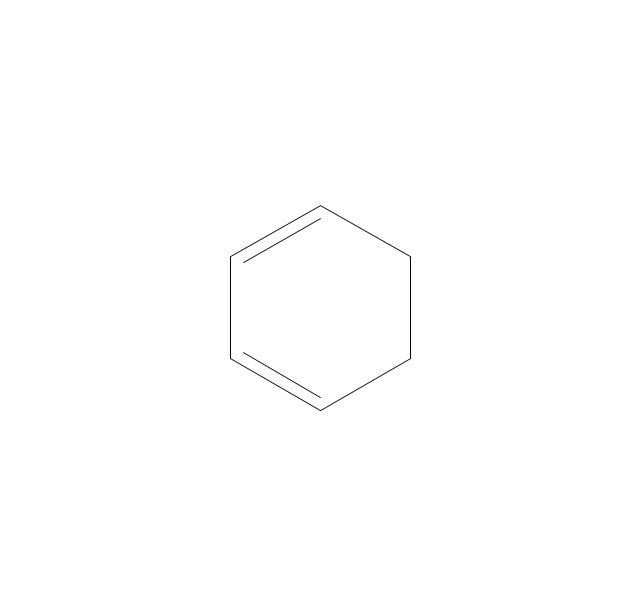
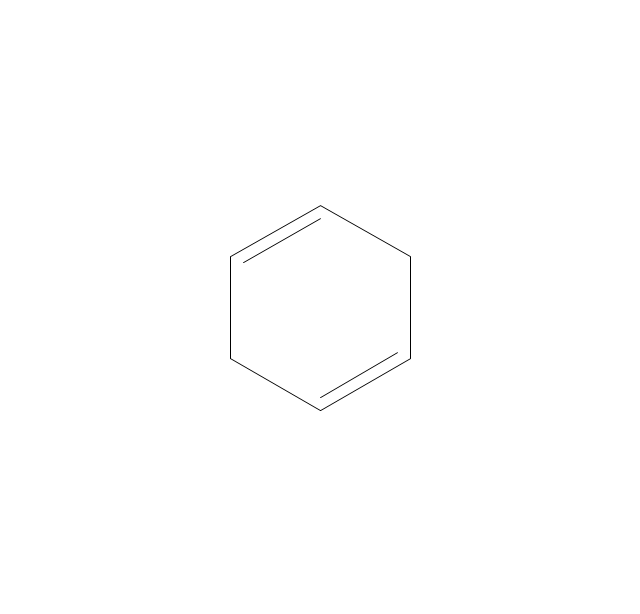
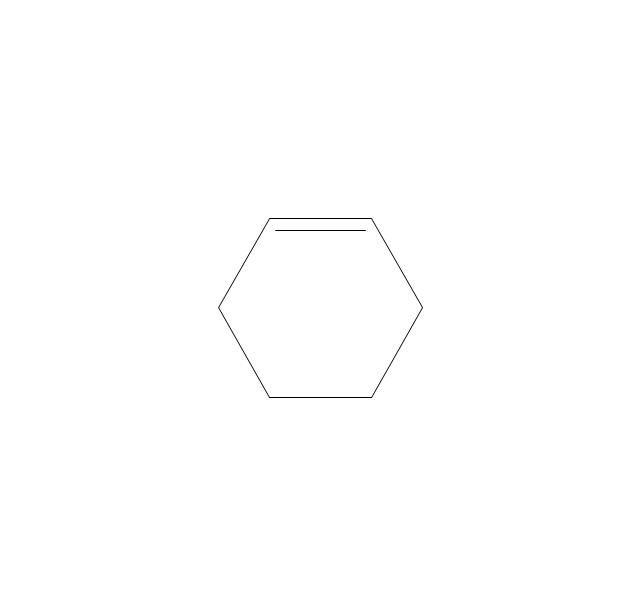
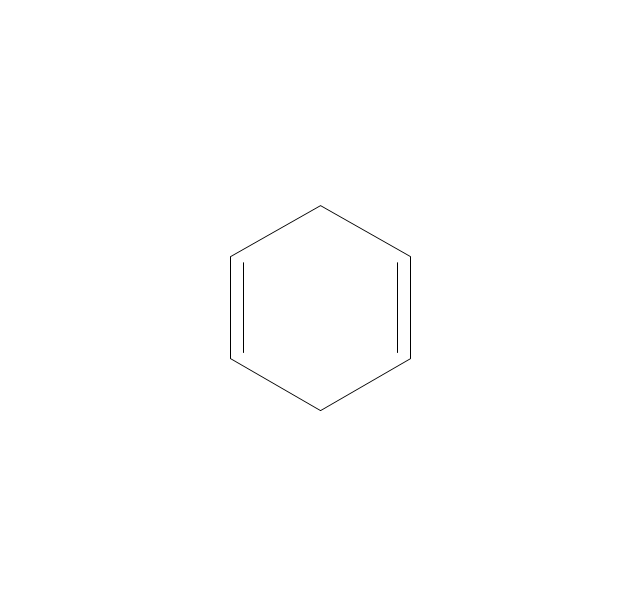
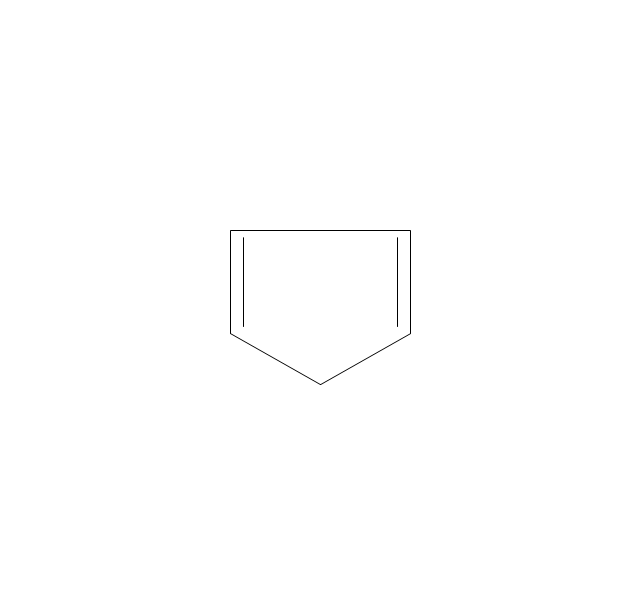
The vector stencils library "Aromatics" contains 23 symbols of aromatic rings for chemical drawing of molecular structural formulas and reaction mechanism schemes in organic chemistry.
"In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. ... Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization. ... Types of aromatic compounds. The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons. 1. Neutral homocyclics. Benzene, as well as most other annulenes (cyclodecapentaene excepted) with the formula CnHn where n is an even number, such as cyclotetradecaheptaene. 2. Heterocyclics. In heterocyclic aromatics (heteroaromats), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine, pyrazine, imidazole, pyrazole, oxazole, thiophene, and their benzannulated analogs (benzimidazole, for example). 3. Polycyclics. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings). Examples are naphthalene, anthracene, and phenanthrene. 4. Substituted aromatics. Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin), paracetamol, and the nucleotides of DNA. 5. Atypical aromatic compounds. Aromaticity is found in ions as well: the cyclopropenyl cation (2e system), the cyclopentadienyl anion (6e system), the tropylium ion (6e), and the cyclooctatetraene dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as tropone. Aromatic properties are tested to the limit in a class of compounds called cyclophanes. A special case of aromaticity is found in homoaromaticity where conjugation is interrupted by a single sp³ hybridized carbon atom. When carbon in benzene is replaced by other elements in borabenzene, silabenzene, germanabenzene, stannabenzene, phosphorine or pyrylium salts the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized. Hexasilabenzene (Si6H6) and borazine (B3N3H6) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring." [Aromaticity. Wikipedia]
The organic compound structural formulas example "Aromatics - Vector stencils library" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. ... Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization. ... Types of aromatic compounds. The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons. 1. Neutral homocyclics. Benzene, as well as most other annulenes (cyclodecapentaene excepted) with the formula CnHn where n is an even number, such as cyclotetradecaheptaene. 2. Heterocyclics. In heterocyclic aromatics (heteroaromats), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine, pyrazine, imidazole, pyrazole, oxazole, thiophene, and their benzannulated analogs (benzimidazole, for example). 3. Polycyclics. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings). Examples are naphthalene, anthracene, and phenanthrene. 4. Substituted aromatics. Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin), paracetamol, and the nucleotides of DNA. 5. Atypical aromatic compounds. Aromaticity is found in ions as well: the cyclopropenyl cation (2e system), the cyclopentadienyl anion (6e system), the tropylium ion (6e), and the cyclooctatetraene dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as tropone. Aromatic properties are tested to the limit in a class of compounds called cyclophanes. A special case of aromaticity is found in homoaromaticity where conjugation is interrupted by a single sp³ hybridized carbon atom. When carbon in benzene is replaced by other elements in borabenzene, silabenzene, germanabenzene, stannabenzene, phosphorine or pyrylium salts the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized. Hexasilabenzene (Si6H6) and borazine (B3N3H6) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring." [Aromaticity. Wikipedia]
The organic compound structural formulas example "Aromatics - Vector stencils library" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
The vector stencils library "Aromatics" contains 23 symbols of aromatic rings for chemical drawing of molecular structural formulas and reaction mechanism schemes in organic chemistry.
"An aromatic hydrocarbon or arene (or sometimes aryl hydrocarbon) is a hydrocarbon with alternating double and single bonds between carbon atoms forming rings. The term 'aromatic' was assigned before the physical mechanism determining aromaticity was discovered, and was derived from the fact that many of the compounds have a sweet scent. The configuration of six carbon atoms in aromatic compounds is known as a benzene ring, after the simplest possible such hydrocarbon, benzene. Aromatic hydrocarbons can be monocyclic (MAH) or polycyclic (PAH)." [Aromatic hydrocarbon. Wikipedia]
The chemical symbols example "Design elements - Aromatic hydrocarbons (arenes)" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"An aromatic hydrocarbon or arene (or sometimes aryl hydrocarbon) is a hydrocarbon with alternating double and single bonds between carbon atoms forming rings. The term 'aromatic' was assigned before the physical mechanism determining aromaticity was discovered, and was derived from the fact that many of the compounds have a sweet scent. The configuration of six carbon atoms in aromatic compounds is known as a benzene ring, after the simplest possible such hydrocarbon, benzene. Aromatic hydrocarbons can be monocyclic (MAH) or polycyclic (PAH)." [Aromatic hydrocarbon. Wikipedia]
The chemical symbols example "Design elements - Aromatic hydrocarbons (arenes)" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
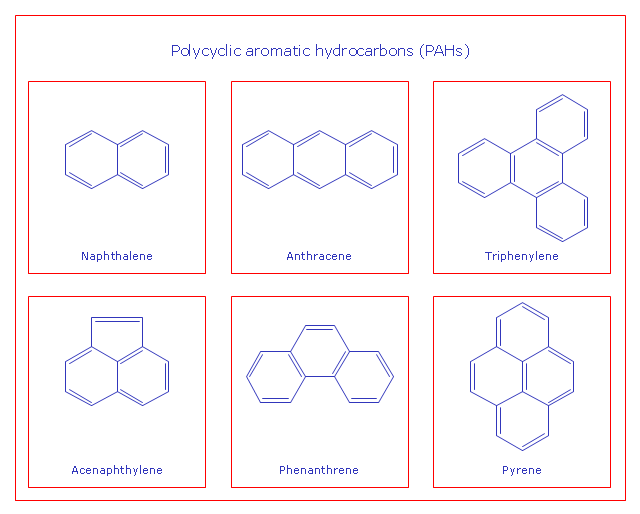
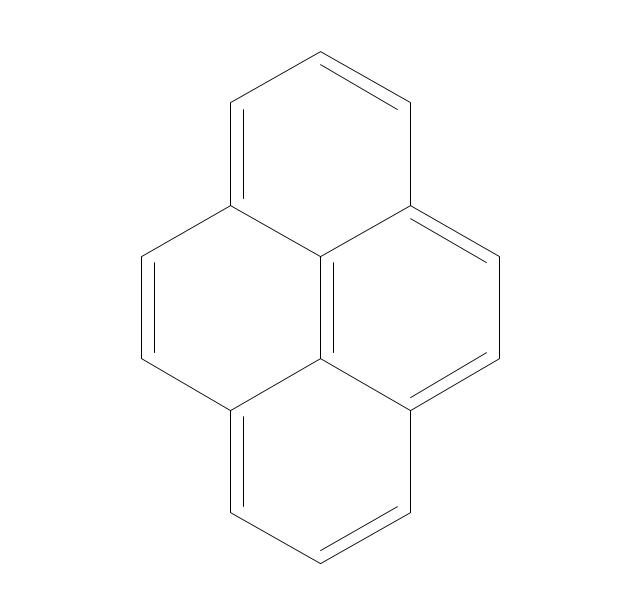
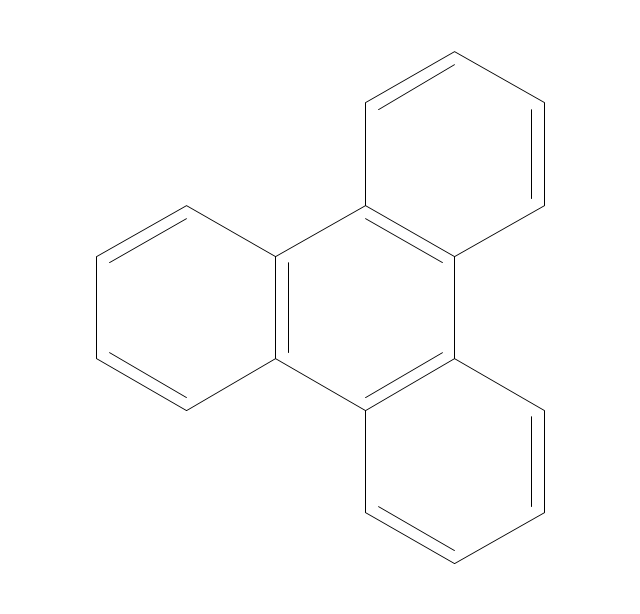
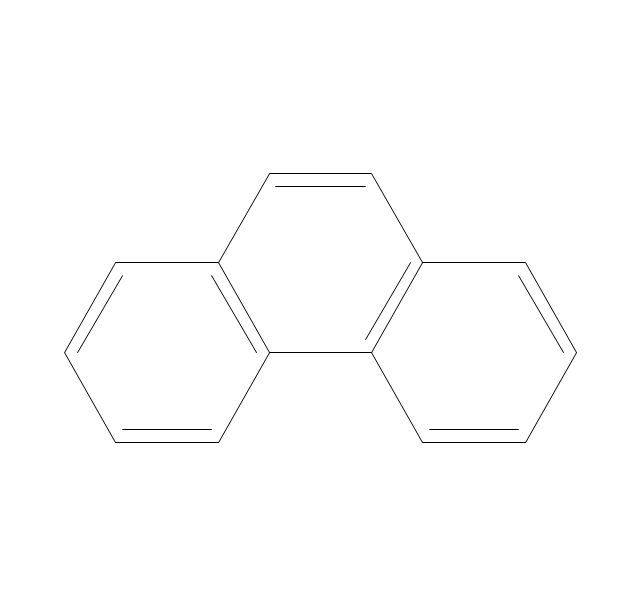
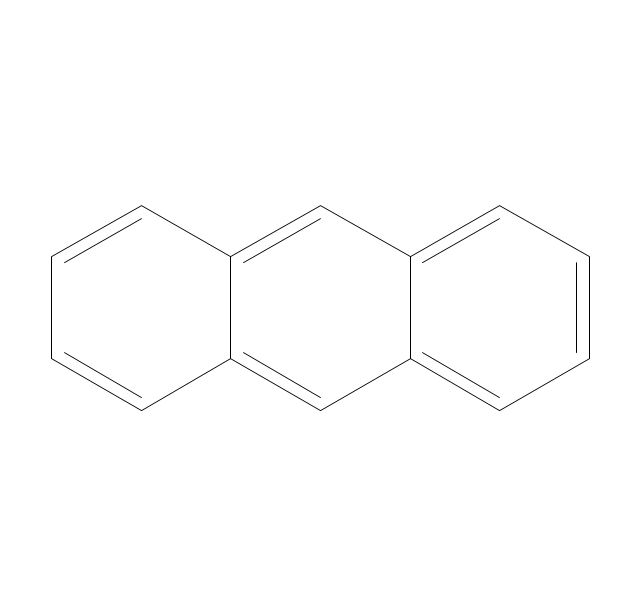
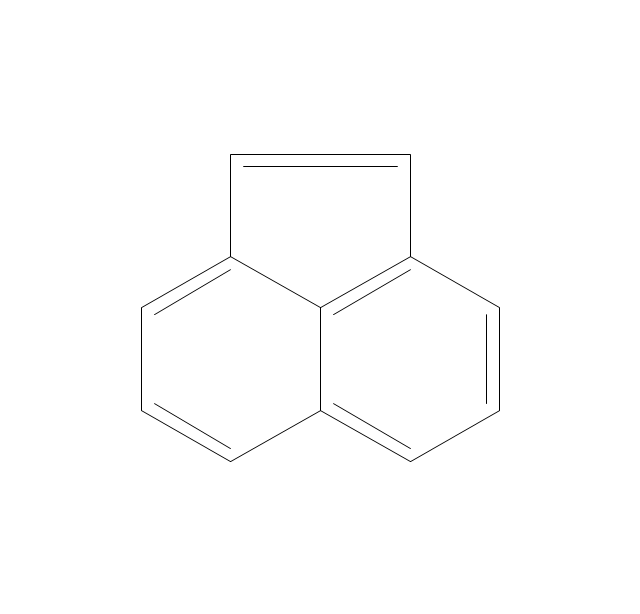
This chemical drawing example shows structural formulas of organic molecules - polycyclic aromatic hydrocarbons (PAHs), also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons: naphthalene, anthracene, triphenylene, acenaphthylene, phenanthrene, pyrene.
"Polycyclic aromatic hydrocarbons (PAHs), also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH. PAHs occur in oil, coal, and tar deposits, and are produced as byproducts of fuel burning (whether fossil fuel or biomass).
They are potent atmospheric pollutants. Some compounds have been identified as carcinogenic, mutagenic, and teratogenic. PAHs are also found in cooked foods. Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures such as grilling or barbecuing, and in smoked fish.
They are also found in the interstellar medium, in comets, and in meteorites and are a candidate molecule to act as a basis for the earliest forms of life." [Polycyclic aromatic hydrocarbon. Wikipedia]
The chemical drawing example "PAH compounds" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"Polycyclic aromatic hydrocarbons (PAHs), also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH. PAHs occur in oil, coal, and tar deposits, and are produced as byproducts of fuel burning (whether fossil fuel or biomass).
They are potent atmospheric pollutants. Some compounds have been identified as carcinogenic, mutagenic, and teratogenic. PAHs are also found in cooked foods. Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures such as grilling or barbecuing, and in smoked fish.
They are also found in the interstellar medium, in comets, and in meteorites and are a candidate molecule to act as a basis for the earliest forms of life." [Polycyclic aromatic hydrocarbon. Wikipedia]
The chemical drawing example "PAH compounds" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
 Chemistry
Chemistry
This solution extends ConceptDraw PRO software with samples, template and libraries of vector stencils for drawing the Chemistry Illustrations for science and education.
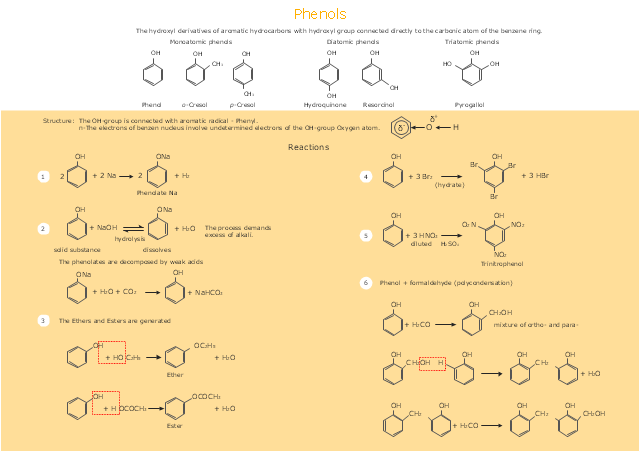
This drawing illustrates examples o f phenolic compounds molecular structures, and chemical reactions of phenols.
"In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (-OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. ...
Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).
Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring." [Phenols. Wikipedia]
The chemical drawing example "Phenols" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (-OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. ...
Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).
Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring." [Phenols. Wikipedia]
The chemical drawing example "Phenols" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
- Aromaticity Examples
- Design elements - Aromatic hydrocarbons (arenes) | Aromatics ...
- Design elements - Aromatic hydrocarbons (arenes) | Arenes
- Phenols | Aromatics - Vector stencils library | Chemistry | Chemical ...
- Non Benzenoid Aromatic Compounds
- Chemistry | Chemistry Drawing Software | Organic Chemistry ...
- Compounds Drawing
- Example Of Elements In Chemistry
- Organic Chemistry Symbols | Design elements - Chemical drawings ...
- Example Of Science Formulas
- Organic Chemistry Symbols | Design elements - Chemical drawings ...
- Design elements - Chemical drawings | Technical Drawing Software ...
- Organic Chemistry Symbols | Physics Symbols | Chemistry Equation ...
- Chemistry Symbols and Meanings | Chemistry Drawing Software ...
- Chemistry Symbols and Meanings | Design elements - Chemical ...
- How to Draw Chemistry Structures | Design elements - Chemical ...
- One Half Examples
- Chemistry Drawings | Chemistry Drawing Software | Chemistry ...
- Pyramid Chart Examples | Dna model of seduction - Triangular ...
- Pyramid Chart Examples | Medicine and health pictograms - Vector ...
.png--diagram-flowchart-example.png)