Accounting Flowcharts
Accounting Flowcharts
Accounting Flowcharts solution extends ConceptDraw PRO software with templates, samples and library of vector stencils for drawing the accounting flow charts.
 Flowcharts
Flowcharts
The Flowcharts Solution for ConceptDraw PRO v10 is a comprehensive set of examples and samples in several different color themes for professionals that need to graphically represent a process. Solution value is added by basic flow chart template and shapes' library of Flowchart notation. ConceptDraw PRO flow chart creator lets one depict a processes of any complexity and length, as well design of the flowchart either vertically or horizontally.
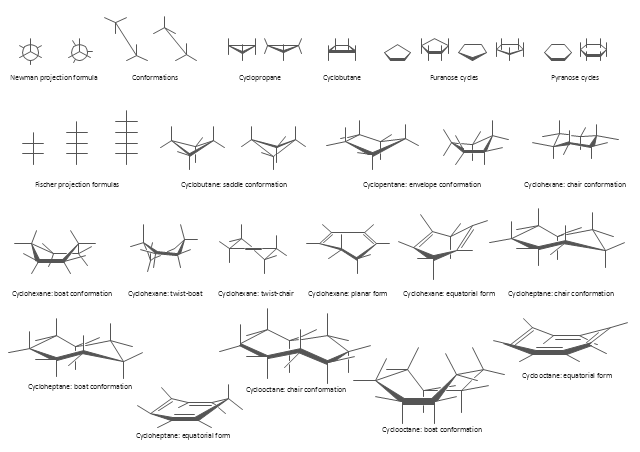
The vector stencils library "Conformations" contains 32 symbols of ring conformations, Newman and Fisher projections for chemical and biochemical drawing the molecular models and structural formulas of organic molecules and biochemical metabolites. It is useful in stereochemistry for drawing spatial structures of conformers of organic molecules, and schemes of stereospecific chemical reactions in organic synthesis.
"In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds (refer to figure on single bond rotation). Such isomers are generally referred to as conformational isomers or conformers and, specifically, as rotamers. Rotations about single bonds are restricted by a rotational energy barrier which must be overcome to interconvert one conformer to another. Conformational isomerism arises when the rotation about a single bond is relatively unhindered. That is, the energy barrier must be small enough for the interconversion to occur.
Conformational isomers are thus distinct from the other classes of stereoisomers (i. e. configurational isomers) where interconversion necessarily involves breaking and reforming of chemical bonds. For example, L- & D and R- & S- configurations of organic molecules have different handedness and optical activities, and can only be interconverted by breaking one or more bonds connected to the chiral atom and reforming a similar bond in a different direction or spatial orientation.
The study of the energetics between different rotamers is referred to as conformational analysis. It is useful for understanding the stability of different isomers, for example, by taking into account the spatial orientation and through-space interactions of substituents. In addition, conformational analysis can be used to predict and explain product(s) selectivity, mechanisms, and rates of reactions." [Conformational isomerism. Wikipedia]
The chemical symbols example "Design elements - Conformations" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds (refer to figure on single bond rotation). Such isomers are generally referred to as conformational isomers or conformers and, specifically, as rotamers. Rotations about single bonds are restricted by a rotational energy barrier which must be overcome to interconvert one conformer to another. Conformational isomerism arises when the rotation about a single bond is relatively unhindered. That is, the energy barrier must be small enough for the interconversion to occur.
Conformational isomers are thus distinct from the other classes of stereoisomers (i. e. configurational isomers) where interconversion necessarily involves breaking and reforming of chemical bonds. For example, L- & D and R- & S- configurations of organic molecules have different handedness and optical activities, and can only be interconverted by breaking one or more bonds connected to the chiral atom and reforming a similar bond in a different direction or spatial orientation.
The study of the energetics between different rotamers is referred to as conformational analysis. It is useful for understanding the stability of different isomers, for example, by taking into account the spatial orientation and through-space interactions of substituents. In addition, conformational analysis can be used to predict and explain product(s) selectivity, mechanisms, and rates of reactions." [Conformational isomerism. Wikipedia]
The chemical symbols example "Design elements - Conformations" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
- Steps in the Accounting Process | How to Create Flowcharts for an ...
- Steps of Accounting Cycle | What is the Accounting Cycle ...
- Steps of Accounting Cycle | What is the Accounting Cycle ? | Steps in ...
- Cycle Graphic Maker Free
- Steps of Accounting Cycle | Accounting Flowchart: Purchasing ...
- Purchasing Cycle Accounting Information System Flowchart
- Steps of Accounting Cycle | Circular Flow Diagram Template | What ...
- Steps of Accounting Cycle | Pie Chart Examples and Templates ...
- What is the Accounting Cycle ? | Steps of Accounting Cycle ...
- Steps of Accounting Cycle | What is the Accounting Cycle ? | Process ...
- What is the Accounting Cycle ? | Steps of Accounting Cycle ...
- Steps in the Accounting Process | Steps of Accounting Cycle ...
- Steps in the Accounting Process | What is the Accounting Cycle ...
- Steps of Accounting Cycle | What is the Accounting Cycle ? | Circular ...
- What is the Accounting Cycle ? | Steps of Accounting Cycle | Product ...
- Steps of Accounting Cycle | Process Flowchart | Flow Chart Creator ...
- Accounting Flowcharts | What is the Accounting Cycle ? | Rapid UML ...
- What is the Accounting Cycle ?
- Strategic planning - Cycle diagram | Steps of Accounting Cycle ...
- Steps of Accounting Cycle | Workflow to Make a Purchase ...