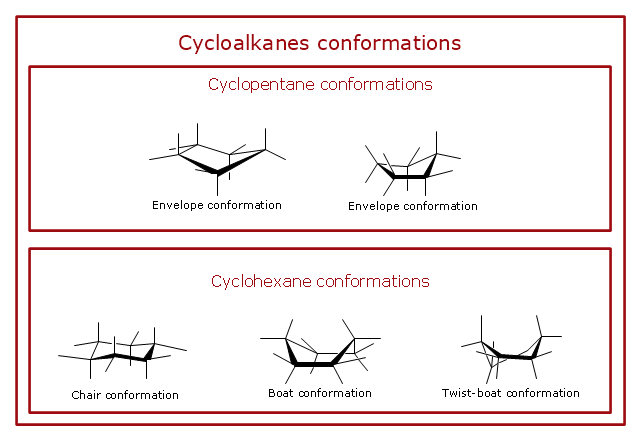
"A cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds.
The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them - chair and twist-boat - can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry because they are the classical example of conformational isomerism and have noticeable influence on the physical and chemical properties of cyclohexane." [Cyclohexane conformation. Wikipedia]
The chemical drawing example "Cycloalkanes conformations" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them - chair and twist-boat - can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry because they are the classical example of conformational isomerism and have noticeable influence on the physical and chemical properties of cyclohexane." [Cyclohexane conformation. Wikipedia]
The chemical drawing example "Cycloalkanes conformations" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"A cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds.
The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them - chair and twist-boat - can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry because they are the classical example of conformational isomerism and have noticeable influence on the physical and chemical properties of cyclohexane." [Cyclohexane conformation. Wikipedia]
The chemical drawing example "Cycloalkanes conformations" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them - chair and twist-boat - can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry because they are the classical example of conformational isomerism and have noticeable influence on the physical and chemical properties of cyclohexane." [Cyclohexane conformation. Wikipedia]
The chemical drawing example "Cycloalkanes conformations" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
- Conformations - Vector stencils library | Conformations - Vector ...
- Conformations - Vector stencils library | Venn Diagram Examples for ...
- Conformations - Vector stencils library | Electrical Drawing Software ...
- Conformations - Vector stencils library | Draw The Structure Of ...
- Cycloalkanes conformations | Design elements - Conformations ...
- Cycloalkanes conformations | Cyclopentane Half Chair
- Sofas and chairs - Vector stencils library | Office furniture - Vector ...
- Office furniture - Vector stencils library | Sofas and chairs - Vector ...
- Cycloalkanes conformations | Planar Hexagon Conformation Of ...
- Cycloalkanes conformations | Sunrooms - Vector stencils library ...